2D Gel electrophoresis.
The electrophoretic separation of proteins using polyacrylamide gels is a well established biochemical technique. The gels are formed by polymerizing a mixture of acrylamide and bis-acrylamide in the presence of a buffered solution to give a cross-linked polyacrylamide matrix. A mixture of proteins is applied to the gel, and are separated by an applied external electric field. If the gel is cast in a buffer system chosen such that a pH gradient is formed by the electric field, the proteins will migrate to the point in the gel where the pH is equal to their isoelectric point, and this technique is appropriately called isoelectric focussing. Alternatively, the sample can be pre-treated by boiling in the presence of the anionic amphiphile sodium dodecyl sulphate (SDS), where it is assumed that the numbers of SDS molecules bound is proportional to the size of the proteins. If this is the case, then in theory the mass/charge ratio will be same for all proteins, and so the mobility during polyacrylamide gel electrophoresis (PAGE) will be determined by the degree of hindrace offered by the gel matrix. Therefore smaller proteins will migrate more rapidly and the protein mixture is separated according to size. In reality, various factors such as the shape of the protein molecule or anomalous SDS affinity may contribute to protein mobility in SDS-PAGE.
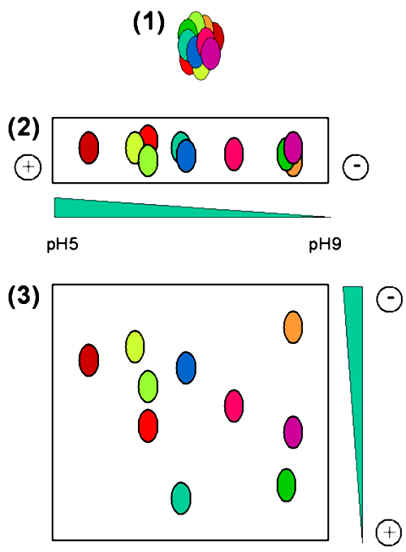
During both isoelectric focussing and SDS-PAGE, the proteins migrate in essentially a linear way, and so these techniques can be considered as one dimensional (1D). As shown below, in 2D gel electrophoresis, a complex protein mixture (illustrated in step 1) is first separated by isoelectric focussing along a pH gradient on a narrow gel (step 2). This narrow gel is then overlaid on one edge of a slab gel, and subjected to SDS-PAGE using a perpendicular electric field (shown in step 3). In this way complex polypeptide mixtures may be analysed to separate the individual proteins.
After the 2D gel has been run, the proteins must be located within the gel, and a variety of protein staining protocols are available. The staining procedure to be used must be compatible with any subsequent operations that are planned, since the compounds added during the staining procedure may act as contaminants during the subsequent mass spectrometry analysis. The staining proceeds of choice involves the use of Coomassie Blue, which is not only a cheap and rapid procedure, but also has the advantage that the stain does not interfere with subsequent protein analysis by mass spectrometry.