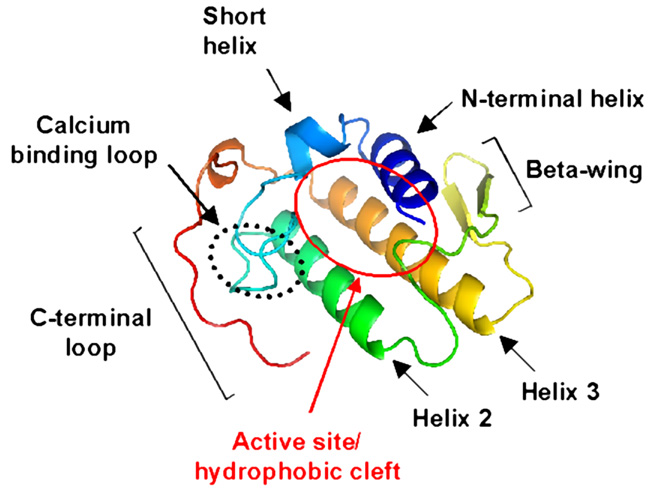
3D structure of Group I/II phospholipases A2.
The PLA2s belonging to groups I and II have highly similar amino acid sequences, and X-ray crystallography studies have shown that their three-dimensional structures are also highly conserved. As shown in the the figure below, two anti-parallel disulfide linked alpha helices (helices 2 and 3) define a rigid scaffold to which the calcium binding loop, the C-terminal loop and a single double stranded beta sheet (the so-called "beta wing") are covalently linked by disulfide bridges. In group II PLA2s the position of the N-terminal helix (labeled as helix 1 in the figure) is stabilized by extensive side chain contacts with both beta wing and the body of the protein.
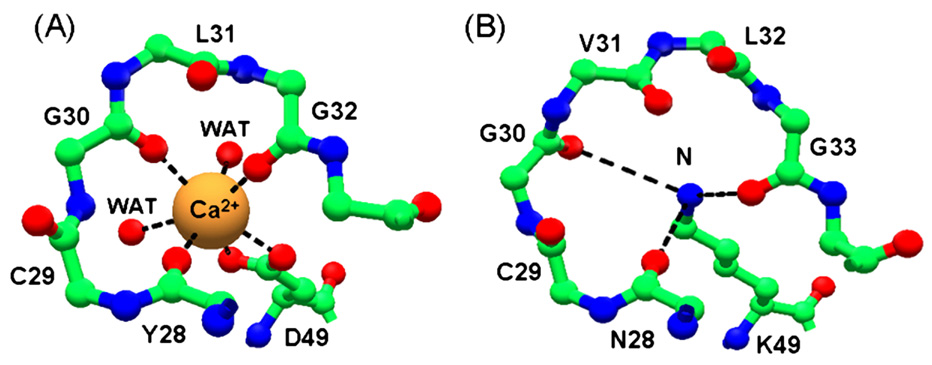
One of our interests is the study of membrane damage by a sub-family of group II PLA2s, in the aspartic acid at position 49 in the catalytic site is substituted by a lysine. In the crystal structures of lysine49 PLA2s, the epsilon nitrogen atom of the lysine 49 (shown on the right, and labelled B, in the figure below) is located exactly that the position occupied by the calcium ion in the catalytic the active Asp49 PLA2s (shown of the left, labelled A). The positions of the other residues involved in catalytic activity are fully conserved in the lysine 49 PLA2 crystal structures, and the observed loss of catalytic activity in these proteins may be due to steric hindrance of the calcium binding due to this lysine 49 site chain.